
Bio
In 1997, I received my Bachelor's degree in Chemical Sciences from the Faculty of Exact and Natural Sciences (FCEN) at the University of Buenos Aires (UBA). Subsequently, I completed my doctoral thesis at the National Atomic Energy Commission under the supervision of Drs. Roberto Fernández Prini and Hugo Bianchi, studying electrolyte equilibria in aqueous solutions at high temperatures using UV-visible spectrophotometry, and received my Doctorate from the University of Buenos Aires in 2003.
Afterwards, I undertook two postdoctoral stays at the University of Delaware (USA) between 2003 and 2006. First, I worked in Dr. Robert Wood's laboratory on the thermodynamics of electrolytes, and later in Dr. Andrew Teplyakov's laboratory, redirecting my research towards surface physical chemistry. There, I prepared surfaces modified with monolayers and thin layers of organic compounds and characterized them, particularly through spectroscopic measurements.
Upon returning to Argentina in 2007, I began working at the Institute of Physical Chemistry of Materials, Environment, and Energy (INQUIMAE) at FCEN-UBA, within the Molecular Electrochemistry group led by Dr. Ernesto Calvo.
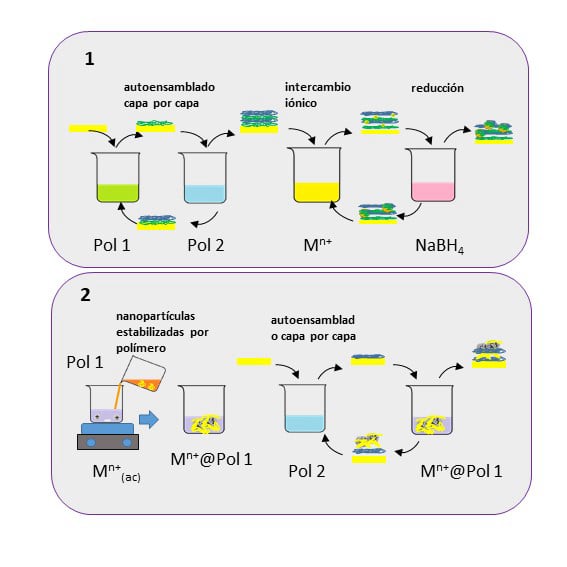
Currently, my research focuses on the preparation and study of nanostructured surfaces using electrochemical, spectroscopic, and spectroelectrochemical techniques, among others. In particular, we implemented the PMIRRAS technique, to which an electrochemical cell was coupled, allowing PMIRRAS spectroelectrochemical experiments and subtractively normalized interfacial infrared spectroscopy (SNIFTIRS).
I am an Independent Researcher at the National Scientific and Technical Research Council (CONICET) and an Associate Professor in the Department of Inorganic, Analytical, and Physical Chemistry at FCEN, UBA.

Research Gate
Orcid
Scopus
CONICET